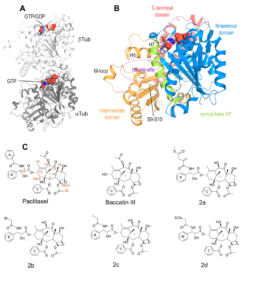
Structural insight into the stabilization 1 of microtubules by taxanes
Paclitaxel (Taxol®) is a taxane and a first-line chemotherapeutic drug that stabilizes microtubules. While the interaction of paclitaxel with microtubules is well described, the current lack of high-resolution structural information on a tubulin-taxane complex precludes a comprehensive description of the binding determinants that affect the drug’s mechanism of action. Here, we solved the crystal structure of the core baccatin III moiety of paclitaxel lacking the C13 side chain in complex with tubulin at 1.9 Å resolution. Based on this information, we engineered two tailor-made taxanes with modified C13 side chains, solved their crystal structures in complex with tubulin, and analyzed their effects along with those of paclitaxel, docetaxel, and baccatin III on the microtubule lattice by X-ray fiber diffraction. We then compared high-resolution structures of ligand-bound tubulin and microtubule complexes with apo forms and used molecular dynamics simulations to understand the consequences of taxane binding to tubulin as well as to simplified protofilament and microtubule-lattice models. Our combined approach sheds light on three mechanistic questions. Firstly, taxanes bind better to microtubules as compared to unassembled tubulin due to a dual structural mechanism: Tubulin assembly is linked to a conformational reorganization of the bM loop, which otherwise occludes ligand access to the taxane site, while the bulky C13 side chains preferentially recognize
the microtubule-assembled over the unassembled conformational state of tubulin. Second, the occupancy of the taxane site by a ligand has no influence on the straightness of tubulin protofilaments. Finally, binding of the taxane core to the taxane site displaces the S9-S10 loop of b-tubulin resulting in microtubule expansion. Our results provide
detailed new insights into the mechanism of microtubule-stabilization by taxanes.
https://www.tubintrain.eu/wp-content/uploads/2023/03/2021.07.20.453061v2.full_-1.pdf
Computational Approaches to the Rational Design of Tubulin-Targeting Agents
Microtubules are highly dynamic polymers of α,β-tubulin dimers which play an essential role in numerous cellular processes such as cell proliferation and intracellular transport, making them an attractive target for cancer and neurodegeneration research. To date, a large number of known tubulin binders were derived from natural products, while only one was developed by rational structure-based drug design. Several of these tubulin binders show promising in vitro profiles while presenting unacceptable off-target effects when tested in patients. Therefore, there is a continuing demand for the discovery of safer and more efficient tubulin-targeting agents. Since tubulin structural data is readily available, the employment of computer-aided design techniques can be a
key element to focus on the relevant chemical space and guide the design process. Due to the high diversity and quantity of structural data available, we compiled here a guide to the accessible tubulin-ligand structures. Furthermore, we review different ligand and structure-based methods recently used for the successful selection and design of new tubulin-targeting agents.
https://www.tubintrain.eu/wp-content/uploads/2023/02/biomolecules-13-00285.pdf
Maytansinol Functionalization: Towards Useful Probes for Studying Microtubule Dynamics
Maytansinoids continue to excite interest almost after a decade after their discovery as potent antimitotic tubulin binders.[1–3] Their highly efficient mechanism of capping MT-dynamics[4] has led to their successful application in cancer treatment as antibody-drug conjugates (ADCs).[5–7] This success has further prompted their development as targeted cancer therapeutics, for example, in the form of nanoparticles[8,9] or recently reported immune checkpoint-targeting maytansinoid conjugates.[10] However, the high-affinity of maytansine towards β-tubulin (KD=6.8–14 nM) not only makes it well suited as a cytotoxin, but also derivatives with a similar affinity could be used as molecular probes.[11,12] As of today, effortless generation of maytansine-based molecular probes is hampered by major drawbacks such as the complexity of the natural product scaffold and lack of SAR studies, which could suggest suitable points for attachment of fluorophore tags or radionuclides. Here, we report on both of these aspects: the chemistry of maytansinol (Figure 1, 1) for the creation of maytansinoid conjugates and the suitability of the C3-position to tolerate bulky substituents without affecting the binding mode. Therefore,
we provide a solid basis for in-depth exploration of maytansinoids as molecular probes to study microtubule dynamics. Previously, it has been shown that the ester at the C-3 position of ansamitocins, maytansine, and maytansinoids plays an important role in biological activity and cell permeability.[3,13,14] In our recent study, we extensively investigated a series of C-3 derivatized maytansinoids obtained by acylation of maytansinol.[12] By X-ray crystallography experiments, we observed that the studied maytansinoids retained a fundamental spatial arrangement with respect to β-tubulin. The binding mode of the core structure of maytansinol remained unaltered independently of the introduced substituents. Furthermore, the binding affinities of the C3-substituted maytansinoids obtained are very similar to the one of maytansine. Based on these observations, we decided to further investigate the potential of C-3 functionalized maytansinoids as molecular probes by creating novel tubulin binders. The design of novel maytansinoids was led by the high-resolution X-ray
crystallography structures of recently obtained maytansinoidtubulin complexes (PDB IDs: 5SB9, 5SBA and 5SBB). The maytansine binding site is located in proximity of the GDP nucleotide bound to the E-site and a GDP-coordinated Mg2+. The first interesting factor that could be exploited to exert a
novel effect on MTs is the Mg2+ ion coordinated by the nucleotide in close proximity to the maytansine binding site. The design of bivalent compounds containing maytansinol and nucleotide mimetics would a) increase the understanding of the ability of maytansinoids to accommodate in the binding site
independently of the size of the substituents, and b) investigate the ability of these molecules to interact with Mg2+ and to displace the nucleotide from the binding site. Tubulin inhibition by nucleotide analogues is very challenging because of GTP concentrations around 300 μM inside the cells.[15] However, we sought to investigate whether the presence of the maytansinol moiety in a bivalent compound could favor binding of the nucleotide portion through an
entropic effect. The maytansinol moiety would act as an anchor point holding the modified nucleotide portion in close proximity of the E-site through a flexible linker, thereby favoring the nucleotide exchange. Accordingly, we decided to prepare long-chain maytansinol derivatives and maytansinoid conjugates to target either the Mg2+ ion or the nucleotide binding site. Introducing different ubstituents at the 3-O position should allow the protein-ligand
interaction to be optimized and new molecules capable of producing different effects on tubulin structure and dynamics to be found
Microtubule-modulating Agents in the Fight Against Neurodegeneration: Will it ever Work?
The microtubule skeleton plays an essential role in nerve cells as the most important structural determinant of morphology and as a highway for axonal transport processes. Many neurodegenerative diseases are characterized by changes in the structure and organization of microtubules and microtubule-regulating proteins such as the microtubule-associated protein tau, which exhibits characteristic changes in a whole class of diseases collectively referred to as tauopathies. Changes in the dynamics of microtubules appear to occur early under neurodegenerative conditions and are also likely to contribute to age-related dysfunction of neurons. Thus, modulating microtubule dynamics and correcting impaired microtubule stability can be a useful neuroprotective strategy to counteract the disruption of the microtubule system in disease and aging. In this article, we review current microtubule-directed approaches for the treatment of neurodegenerative diseases with microtubules as a drug target, tau as a drug target, and post-translational modifications as potential modifiers of the microtubule system. We discuss limitations of the approaches that can be traced back to the rather unspecific mechanism of action, which causes undesirable side effects in non-neuronal cell types or which are due to the disruption of non-microtubule-related interactions. We also develop some
thoughts on how the specificity of the approaches can be improved and what further targets could be used for modulating substances.
https://www.tubintrain.eu/wp-content/uploads/2022/05/Brandt.pdf
Maytansinol derivatives: side reactions as a chance for new tubulin binders
Maytansinol is a valuable precursor for the preparation of maytansine derivatives (known as maytansinoids). Inspired by its intriguing structure and their success in targeted cancer therapy, we explored the maytansinol acylation reaction. As a result, we were able to obtain a series of derivatives, bearing novel modifications of the maytansine scaffold. We characterized these molecules by docking studies, by a comprehensive biochemical evaluation and by
determination of their crystal structures in complex with tubulin. The obtained results shed further light on the intriguing chemical behavior of maytansinoids and confirm the relevance of this peculiar scaffold in the scenario of tubulin binders.
https://www.tubintrain.eu/wp-content/uploads/2021/11/Passarella-Pieraccini-Diaz-Prota.pdf
1,3-Benzodioxole-Modified Noscapine Analogues: Synthesis, Antiproliferative Activity, and Tubulin-Bound Structure
Since the revelation of noscapine’s weak anti-mitotic activity, extensive research has been conducted over the past two decades, with the goal of discovering noscapine derivatives with improved potency. To date, noscapine has been explored at the 1, 7, 6’, and 9’-positions, though the 1,3-benzodioxole motif in the noscapine scaffold that remains unexplored. The present investigation describes the design, synthesis and pharmacological evaluation of noscapine analogues consisting of modifications to the 1,3-benzodioxole moiety. This includes expansion of the dioxolane ring and inclusion of metabolically
robust deuterium and fluorine atoms. Favourable structural modifications were subsequently incorporated into multifunctionalised noscapine derivatives that also possessed modifications previously shown to promote anti-proliferative activity in the 1-, 6’- and 9’-positions. Our research efforts afforded the deuterated noscapine derivative 14e and the dioxino-containing analogue 20 as potent cytotoxic agents with EC50 values of 1.50 and 0.73 μM, respectively, against breast cancer (MCF-7) cells. Compound 20 also exhibited EC50 values of <2 μM against melanoma, non-small cell lung carcinoma, and cancers of the
brain, kidney and breast in an NCI screen. Furthermore, compounds 14e and 20 inhibit tubulin polymerisation and are not vulnerable to the overexpression of resistance conferring Pgp efflux pumps in drug-resistant breast cancer cells (NCIADR/RES). We also conducted X-ray crystallography studies that yielded
the high-resolution structure of 14e bound to tubulin. Our structural analysis revealed the key interactions between this noscapinoid and tubulin and will assist with the future design of noscapine derivatives with improved properties.
https://www.tubintrain.eu/wp-content/uploads/2021/08/Anne-Catherine-cmdc.202100363-3.pdf
Microtubule-targeting agents and neurodegeneration
The association of microtubule (MT) breakdown with neurodegeneration and neurotoxicity has provided an emerging therapeutic approach for neurodegenerative diseases. Tubulin binders are able to modulate MT dynamics and, as a result, are of particular interest both as potential therapeutics and experimental tools used to validate this strategy. Here, we provide a comprehensive overview of current knowledge and recent advancements regarding MT-targeting approaches for neurodegeneration and evaluate the potential application of MT-targeting agents (MTAs) based on available preclinical and clinical data.
https://www.sciencedirect.com/science/article/pii/S135964462030516X